DNA Methylation: How it works
DNA methylation is one of the ways your cells control which genes to express. It is an example of epigenetic modification. Epigenetics mechanisms like this do not change the DNA sequence, only the way the genes are expressed. Whether or not DNA methylation is heritable is not clear.
This is how DNA methylation works:
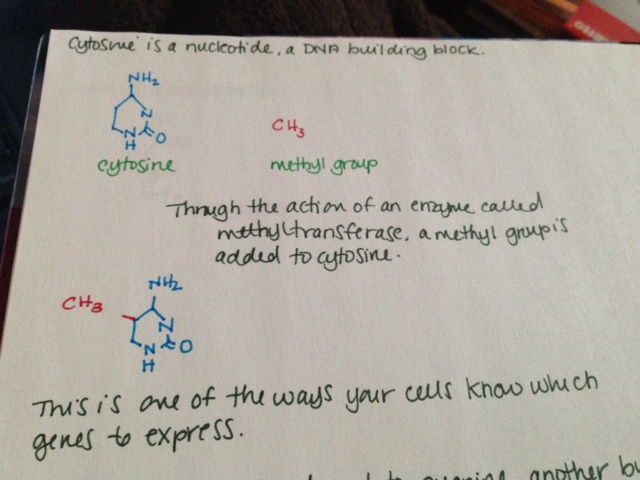
- Cytosine is a nucleotide, a DNA building block.
- Through the action of an enzyme called methyltransferase, a methyl group is added to cytosine.
- This is one of the ways your cells know which genes to express.
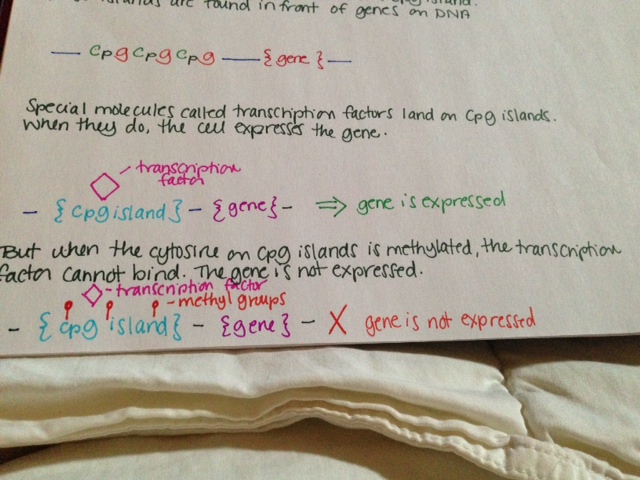
- Cytosine is often found next to guanine, another building block. This is sometimes shown in literature as “CG.”
- Cytosine and guanine are connected by a phosphate group. This is sometimes shown in literature as “CpG.”
- A bunch of CpG sites together is called a CpG island. These islands are found in front of genes on DNA.
- Special molecules called transcription factors land on CpG islands. When they do, the cell expresses the gene.
- But when the cytosine on CpG islands is methylated, the transcription factor cannot bind. The gene is not expressed.
See pictures below.
Methylation is known to have an important role in cancer biology. Methylation of tumor suppressor genes causes the tumor suppressors not to be expressed, resulting in cancer.